Description
Our group has the ability to characterize and cultivate plant and soil microbiomes for beneficial microbial symbionts that can improve crop performance through priming and complementing host disease resistance, improving plant nutrient acquisition, and supplementing host environmental stress responses. We use molecular ecology approaches to understand the diversity and function of crop bacterial and fungal microbiome members and advanced approaches to cultivate these under laboratory conditions for controlled inoculation and growth trials across spatial scales from the laboratory to the field.
Capability Bounds
Multi-omic approaches for characterization of complex microbial community structure and function paired with advanced cultivation approaches can be applied in soil, rhizosphere, and within internal root tissues. Our in house curated culture collection of 5,000 bacterial isolates and 4,000 fungal isolates from different plant hosts includes over 400 genomes from the collection that are genome sequenced.
Unique Aspects
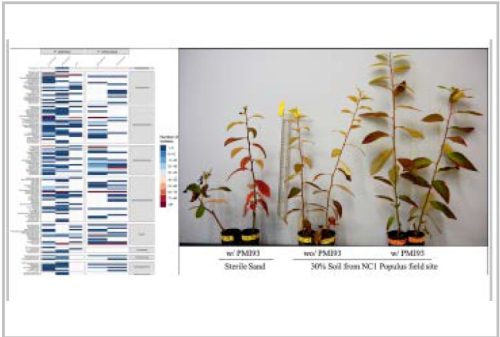
- Ability to characterize microbiomes within intact plant tissues via integrated multi-omic analyses
- Development of targeted and customized microbial isolation and cultivation techniques
- Controlled characterization and crop trials under greenhouse and field conditions that include both physiological and multi-modal plant phenotyping
Availability
Our approaches have been widely tested and applied within woody plant and grass biofeedstocks, but are transferable across a variety of crop species.
Benefit
Microbial symbionts can be applied to enhance plant host nutrient acquisition, improve disease resistance, and increase crop stress tolerance to environmental challenges.
Capability Expert(s)
Christopher Schadt, Alyssa Carrell, Melissa Cregger, Dale Pelletier, Mircea Podar, Tomas Rush, and David Weston
References
Bonito, Gregory, et al. “Isolating a functionally relevant guild of fungi from the root microbiome of Populus.” Fungal Ecology 22 (2016): 35-42.
Carper, Dana L., et al. “Cultivating the bacterial microbiota of Populus roots.” Msystems 6.3 (2021): e01306-20.
Cregger, Melissa A., et al. “Plant–microbe interactions: from genes to ecosystems using Populus as a model system.” Phytobiomes Journal 5.1 (2021): 29-38.
Dove, Nicholas C., et al. “Assembly of the Populus microbiome is temporally dynamic and determined by selective and stochastic factors.” Msphere 6.3 (2021): e01316-20.
Veach, Allison M., et al. “Plant hosts modify belowground microbial community response to extreme drought.” Msystems 5.3 (2020): 10-1128.
Rush TA et al., (2022) Lipo-chitooligosaccharides induce specialized fungal metabolite profiles that modulate bacterial growth (opens in new window).
Carrel AA et al., (2023) A consortium of ectomycorrhizal fungi and a beneficial bacterium differentially regulates carbon sequestration and exometabolites production across Populus trichocarpa genotypes (opens in new window). Plant Direct
Gopalakrishnan Meena M, et al., (2023) A glimpse into the fungal metabolomic abyss: Network analysis for revealing relationships between exogenous compounds and their outputs (opens in new window). PNAS Nexus
